Welcome to the March edition of the JITC Digest. As we approach the end of the month, it means the 2024 American Association for Cancer Research (AACR) Annual Meeting is just around the corner. I look forward to seeing those of you who will be joining me in beautiful San Diego. I also encourage you to visit the SITC booth in the exhibition hall during the conference. Our Deputy Editor-in-Chief, Sjoerd H. van der Burg, will be holding a meet-the-editor session at the booth on Tuesday, April 9th, 11:45 am–12:30 pm PT. It is a great opportunity to discuss your work with him and learn more about the journal.
While I am excited to view the latest research at AACR 2024, there is no shortage of quality offerings each month in JITC. Recent highlights include a four-year follow-up by David P. Carbone et al on the Checkmate 9LA clinical trial in which the survival benefit of nivolumab plus ipilimumab with chemotherapy for patients with metastatic or recurrent non-small cell lung cancer is demonstrated, supporting its use as a first-line treatment.
Jeanne Shen and colleagues utilized an artificial intelligence (AI) model that is able to identify the immune phenotype of the tumor microenvironment based on tumor-infiltrating lymphocyte analysis. Capable of determining inflamed immune phenotype (IIP) status, the AI model found a correlation between IIP prevalence and positive immune checkpoint inhibitor (ICI) treatment outcomes of objective response rate, progression-free survival, and overall survival, suggesting AI-based IIP characterization may serve as a tumor-agnostic prognostic biomarker for ICI therapy response across a range of tumor types.
Be sure to check out these articles and more below!
Regards,
James L. Gulley, MD, PhD, FACP
Interim Editor-in-Chief
Journal for ImmunoTherapy of Cancer
JITC Editor Picks
|
Four-year clinical update and treatment switching-adjusted outcomes with first-line nivolumab plus ipilimumab with chemotherapy for metastatic non-small cell lung cancer in the CheckMate 9LA randomized trial
|
|
David P. Carbone, Tudor-Eliade Ciuleanu, Michael Schenker, Manuel Cobo, Stéphanie Bordenave, Oscar Juan-Vidal, Juliana Menezes, Niels Reinmuth, Eduardo Richardet, Ying Cheng, Hideaki Mizutani, Enriqueta Felip, Bogdan Zurawski, Aurelia Alexandru, Luis Paz-Ares, Shun Lu, Thomas John, Xiaoqing Zhang, Javed Mahmood, Nan Hu, Tuli De, Irene Santi, John R. Penrod, Yong Yuan, Adam Lee, Martin Reck
Journal for ImmunoTherapy of Cancer 2024;12:e008189 (12 February 2024)
RESEARCH

Summary:
This publication is a four-year follow-up on the clinical trial Checkmate 9LA that had previously demonstrated an overall survival (OS) benefit for the use of the immunotherapy (IO) treatment of nivolumab plus ipilimumab with chemotherapy (IO+chemo) in comparison to chemotherapy alone as a first-line treatment for patients with metastatic non-small cell lung cancer (NSCLC). At a minimum of 47.9-month follow-up, the OS of the IO+chemo treatment group in all randomized patients was 21% versus the 16% of chemotherapy-alone group, regardless of tumor PD-L1 expression or histology. Interestingly, the OS of patients who had to discontinue IO+chemo treatment due to treatment-related adverse events was 41%. Finally, treatment-switching analysis on OS data was performed, which lowered the OS of the chemotherapy-alone group. This study further supports the use of nivolumab + ipilimumab with chemotherapy as a first-line treatment for patients with metastatic or recurrent NSCLC.
|
|
Inflamed immune phenotype predicts favorable clinical outcomes of immune checkpoint inhibitor therapy across multiple cancer types
|
|
Jeanne Shen, Yoon-La Choi, Taebum Lee, Hyojin Kim, Young Kwang Chae, Ben W Dulken, Stephanie Bogdan, Maggie Huang, George A Fisher, Sehhoon Park, Se-Hoon Lee, Jun-Eul Hwang, Jin-Haeng Chung, Leeseul Kim, Heon Song, Sergio Pereira, Seunghwan Shin, Yoojoo Lim, Chang Ho Ahn, Seulki Kim, Chiyoon Oum, Sukjun Kim, Gahee Park, Sanghoon Song, Wonkyung Jung, Seokhwi Kim, Yung-Jue Bang, Tony S K Mok, Siraj M. Ali, Chan-Young Ock
Journal for ImmunoTherapy of Cancer 2024;12:e008339 (14 February 2024)
RESEARCH

Summary:
Tumor-infiltrating lymphocytes (TIL) within the tumor microenvironment have been associated with a positive response to immune checkpoint inhibitor (ICI) therapy, however, manual evaluation of TILs is time consuming and labor intensive. To offer a solution, this group developed a deep-learning artificial intelligence (AI) software that determines inflamed immune phenotype (IIP) within tumor samples, defined by enrichment of TILs. The program was trained on 17,296 H&E whole-slide images of over 24 different solid tumor types from 9 different institutions that had been manually annotated by board-certified pathologists. The AI tool was tested on 1,806 pre-treatment samples of ICI-treated patients across 27 solid tumor types. It was capable of determining IIP status of tissue and found a correlation between IIP prevalence and positive ICI treatment outcomes of objective response rate, progression-free survival, and overall survival. In summary, AI-based IIP characterization may represent a practical alternative to manual IIP characterization and prediction of prognosis of ICI therapy response across diverse tumor types.
|
|
CAR-mediated targeting of NK cells overcomes tumor immune escape caused by ICAM-1 downregulation
|
Jiri Eitler, Wiebke Rackwitz, Natalie Wotschel, Venugopal Gudipati, Nivedha Murali Shankar, Anastasia Sidorenkova, Johannes B Huppa, Paola Ortiz-Montero, Corinna Opitz, Stephan R Künzel, Susanne Michen, Achim Temme, Liliana Rodrigues Loureiro, Anja Feldmann, Michael Bachmann, Laurent Boissel, Hans Klingemann, Winfried S Wels, Torsten Tonn
Journal for ImmunoTherapy of Cancer 2024;12:e008155 (27 February 2024)
RESEARCH
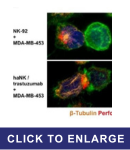
Summary:
Natural killer (NK) cells are effective at killing malignant cells and have been genetically engineered or paired with a therapeutic antibody to enhance their cancer-targeting abilities. One monoclonal antibody that enhances NK cell activity, trastuzumab, is used in breast cancer treatment by targeting ErbB2, which is often overexpressed in breast cancers. Since NK cells work primarily through the LFA-1/ICAM-1 axis, this study showed how downregulation of ICAM-1 in tumors provides an escape mechanism by preventing trastuzumab-mediated NK cell toxicity. Chimeric antigen receptor (CAR)-engineered NK cells override this signaling axis and effectively kill tumor cells regardless of ICAM-1 expression. Additionally, pre-treatment with drugs such as 5-aza-2'-deoxycytidine to induce ICAM-1 upregulation may be a viable option to reinvigorate trastuzumab-mediated NK cell activity. These data demonstrate how NK cells can be leveraged to overcome breast cancer cell resistance to treatment caused by ICAM-1 downregulation and highlight the potential of CAR-NK cells in cancer immunotherapy.
|
|
Targeting the activated microenvironment with endosialin (CD248)-directed CAR-T cells ablates perivascular cells to impair tumor growth and metastasis
|
|
Sarah L Ash, Rebecca Orha, Holly Mole, Meg Dinesh-Kumar, Steven P Lee, Frances K Turrell, Clare M Isacke
Journal for ImmunoTherapy of Cancer 2024;12:e008608 (27 February 2024)
RESEARCH
Summary:
The immunosuppressive tumor microenvironment presents a challenge to chimeric antigen receptor (CAR) T cell therapy particularly due to its lack of suitable tumor-specific antigens to use as a target. The goal of this study was to find a viable alternative target to tumor-specific antigens for CAR T cell therapy in the treatment of solid cancers. The endosialin (CD248) receptor was targeted because it is strongly expressed on tumor-associated pericytes and fibroblasts, which are proximal to tumor vasculature. Using three immunocompetent mouse strains, E3K CAR T cells were generated and transferred into syngeneic mice with breast or lung cancer lines. Target cells were depleted in tumor stroma, driving tumor necrosis, with no detectable on-target/on-tumor effects. These data demonstrate that E3K CAR T cells provide a benefit of avoiding the harsh tumor microenvironment while still effectively inhibiting tumor growth and may be a suitable option as an antigen for CAR T cell therapy for a wide range of solid tumors.
|
Other Recent JITC Articles
JITC Meet-the-Editor Session at AACR
|
|
For those attending the American Association for Cancer Research (AACR) Annual Meeting 2024, visit the SITC booth in the exhibit hall and meet JITC Deputy Editor-in-Chief Dr. Sjoerd H. van der Burg during the JITC Meet-the-Editor Session, 11:45 am–12:30 pm PT on Tuesday, April 9. Stop by to say hello to Dr. van der Burg, share your research, and learn more about JITC. For additional information during AACR 2024, check out JITC’s X/Twitter and LinkedIn profiles. |
Popular Archive Articles
The selections below represent some of the most popular content published in JITC over the past two years. Explore additional thematic content in JITC's Collections or access the rest of JITC's archives for a look at all the journal has to offer.
Safety and antitumor activity of dostarlimab in patients with advanced or recurrent DNA mismatch repair deficient/microsatellite instability-high (dMMR/MSI-H) or proficient/stable (MMRp/MSS) endometrial cancer: interim results from GARNET—a phase I, single-arm study
Ana Oaknin, Lucy Gilbert, Anna V Tinker, Jubilee Brown, Cara Mathews, Joshua Press, Renaud Sabatier, David M O’Malley, Vanessa Samouelian, Valentina Boni, Linda Duska, Sharad Ghamande, Prafull Ghatage, Rebecca Kristeleit, Charles Leath III, Wei Guo, Ellie Im, Sybil Zildjian, Xinwei Han, Tao Duan, Jennifer Veneris, Bhavana Pothuri
Journal for ImmunoTherapy of Cancer 2022;10:e003777 (21 January 2022)
RESEARCH
Gut microbiota and metabolites associate with outcomes of immune checkpoint inhibitor–treated unresectable hepatocellular carcinoma
Pei-Chang Lee, Chi-Jung Wu, Ya-Wen Hung, Chieh Ju Lee, Chen-Ta Chi, I-Cheng Lee, Kuo Yu-Lun, Shih-Hsuan Chou, Jiing-Chyuan Luo, Ming-Chih Hou, Yi-Hsiang Huang
Journal for ImmunoTherapy of Cancer 2022;10:e004779 (23 June 2022)
RESEARCH
Single-cell spatial landscape of immunotherapy response reveals mechanisms of CXCL13 enhanced antitumor immunity
Mark Sorin, Elham Karimi, Morteza Rezanejad, Miranda W Yu, Lysanne Desharnais, Sheri A C McDowell, Samuel Doré, Azadeh Arabzadeh, Valerie Breton, Benoit Fiset, Yuhong Wei, Roni Rayes, Michele Orain, Francois Coulombe, Venkata S K Manem, Andreanne Gagne, Daniela F Quail, Philippe Joubert, Jonathan D Spicer, Logan A Walsh
Journal for ImmunoTherapy of Cancer 2023;11:e005545 (1 February 2023)
RESEARCH
Regulatory implications of ctDNA in immuno-oncology for solid tumors
Paz J Vellanki, Soma Ghosh, Anand Pathak, Michael J Fusco, Erik W Bloomquist, Shenghui Tang, Harpreet Singh, Reena Philip, Richard Pazdur, Julia A Beaver
Journal for ImmunoTherapy of Cancer 2023;11:e005344 (7 February 2023)
REVIEW
|
APC Discounts
As a way to thank the SITC members who work tirelessly to advance the science and improve the lives of cancer patients, SITC will provide members with a 35% discount on Article Processing Charges (APCs) for all JITC articles submitted and accepted through 2024. This discount is applied post-acceptance, at which point a discount code is shared with the corresponding author.
Become a SITC Member Today!
|
|
JITC also offers full waivers for the full APC (100% discount of the APC) where all authors are based in low-income countries (see policy). Requests for waivers must be made prior to submission. For additional information regarding these discounts, as well as institutional arrangements and editor/reviewer discounts, view the journal's APC policy. Additional questions may be directed to JITCEditor@sitcancer.org.
|