On behalf of the Society for Immunotherapy of Cancer (SITC) and in collaboration with the Association for Molecular Pathology (AMP) and the College of American Pathologists (CAP), we are pleased to announce the publication of a new manuscript titled "Recommendations for Tumor Mutational Burden Assay Validation and Reporting: A Joint Consensus Recommendation of the Association for Molecular Pathology, College of American Pathologists, and Society for Immunotherapy of Cancer" in the Journal of Molecular Diagnostics.
Dr. Carlo Bifulco, who serves as Chair of the SITC Pathology Committee, represented the society in this effort to ensure standardization across the field. This manuscript provides recommendations for the analytical validation and reporting of tumor mutational burden (TMB) testing as a potential predictive biomarker for immune checkpoint inhibitor (ICI) therapies. These recommendations encompass pre-analytical, analytical, and post-analytical factors of TMB analysis, and emphasize the importance of comprehensive methodological descriptions in publications to allow comparability between assays.
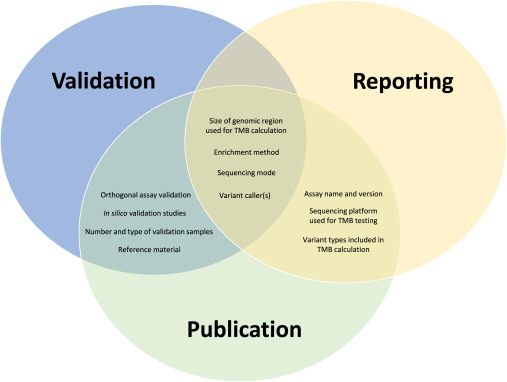
In addition, this manuscript also provides two illustrated supplements, one that summarizes their systematic review of literature describing TMB clinical testing and one that displays recommended elements for inclusion in tumor mutational burden (TMB) validation studies and clinical reports (shown below).